Abstract
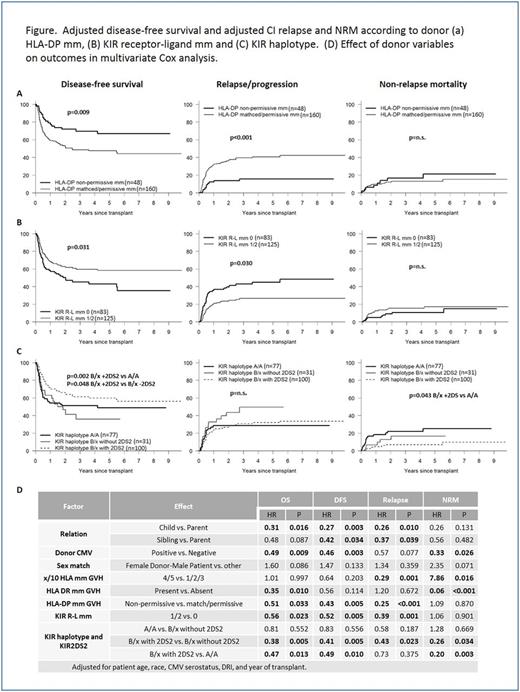
Lack of a matched sibling or unrelated donor can be a significant barrier to allogeneic hematopoietic cell transplantation (HCT). Haploidentical (haplo) donors are readily available for nearly all such patients. However, donor selection criteria to determine the optimal haplo donor are not readily available. In order to determine which donor characteristics are most important in predicting transplant success, we retrospectively analyzed 208 consecutive donor-recipient pairs receiving haplo HCT with post-transplant cyclophosphamide for hematologic malignancy. Donor characteristics were evaluated by multivariate Cox analysis and correlated with overall survival (OS), disease-free survival (DFS), relapse/progression, and non-relapse mortality (NRM), while controlling for significant patient and transplant-related factors. Donor variables analyzed included age, sex, relationship to recipient, CMV status, ABO compatibility, HLA disparity and several NK alloreactivity models (KIR receptor-ligand, ligand-ligand, haplotype, B content, activating KIR-based education systems, Sekine donor licensing model). Median (range) recipient and donor age was 52 (19-75) and 38 (15-73) years respectively, and 41% of donor-recipient pairs were non-Caucasian. Patients were transplanted for AML (34%), MDS/MPS/CML (20%), ALL (17%), NHL/HD/CLL (25%). PBSC was used as the stem cell source in 66% of patients, and conditioning intensity was myeloablative in 41%. The donor was a child, sibling, or parent in 47%, 38%, and 14% respectively. Median (range) follow-up for surviving patients was 33 (7-130) months. In multivariate Cox analysis, patient/transplant characteristics associated with improved OS and DFS included recipient age <55 years, black race, CMV seronegativity, low/intermediate disease risk index (DRI), and more recent transplant year. When adjusting for significant patient/transplant variables, donor characteristics independently associated with improved overall survival included presence of HLA-DR mismatch [GVH direction] (HR 0.35, p=0.010), the presence of HLA DP non-permissive mismatch [GVH direction] (HR 0.51, p=0.033), KIR receptor-ligand mismatch (HR 0.56, p=0.023), the presence of KIR B/x haplotype with KIR2DS2 (HR 0.38, p=0.005 vs. B/x without KIR2DS2; HR 0.47, p=0.013 vs. A/A), donor CMV positivity (HR 0.49, p=0.009) and donor relation (child vs. parent - HR 0.31, p=0.016; sibling vs. parent - HR 0.48, p=0.087). Donor characteristics independently associated with reduced risk of disease relapse/progression included the presence of KIR receptor-ligand mismatch (HR 0.39, p=0.001), KIR B/x haplotype with KIR2DS2 (HR 0.43, p=0.023 vs. B/x without KIR2DS2), the presence of ≥4 (out of 10) HLA allelic mismatches [GVH direction] (HR 0.29, p=0.001), the presence of a non-permissive HLA-DP mismatch (HR 0.25, p<0.001) and the use of a non-parental donor (child vs. parent - HR 0.26, p=0.010; sibling vs. parent - HR 0.37, p=0.039). Donor characteristics associated with increased NRM included higher HLA disparity (HR 7.86, p=0.016), HLA-DR match (HR 15.99, p<0.001), absence of KIR B/x haplotype with KIR2DS2 (A/A haplotype - HR 5.03, p=0.003; B/x without KIR2DS2 - HR 3.92, p=0.034), CMV seronegativity (HR 2.99, p=0.026), and female donor-male recipient (HR 2.35, p=0.071). Adjusted 3-yr OS was improved in patients with the presence of KIR R-L mm (66% vs 50%, p=0.013), KIR B/x with KIR2DS2 (69% vs. 55% [A/A] or 43% [B/x without KIR2DS2, p=0.052 and 0.007, respectively]), HLA-DR mm (64% vs. 45%, p=0.071), and HLA-DP non-permissive mm (72% vs. 56%, p=0.026), emphasizing the importance of donor HLA and KIR typing for optimal donor selection (see figure). This large, single institution analysis demonstrates the significance of HLA-DR/HLA-DP disparity, NK alloreactivity, and other clinical variables in the donor selection process for haplo HCT. These results suggest that HLA-DP and donor KIR typing should be performed routinely in T cell-replete haplo HCT to assist in donor selection and risk stratification.
Solh: ADC Therapeutics: Research Funding; Amgen: Speakers Bureau; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal